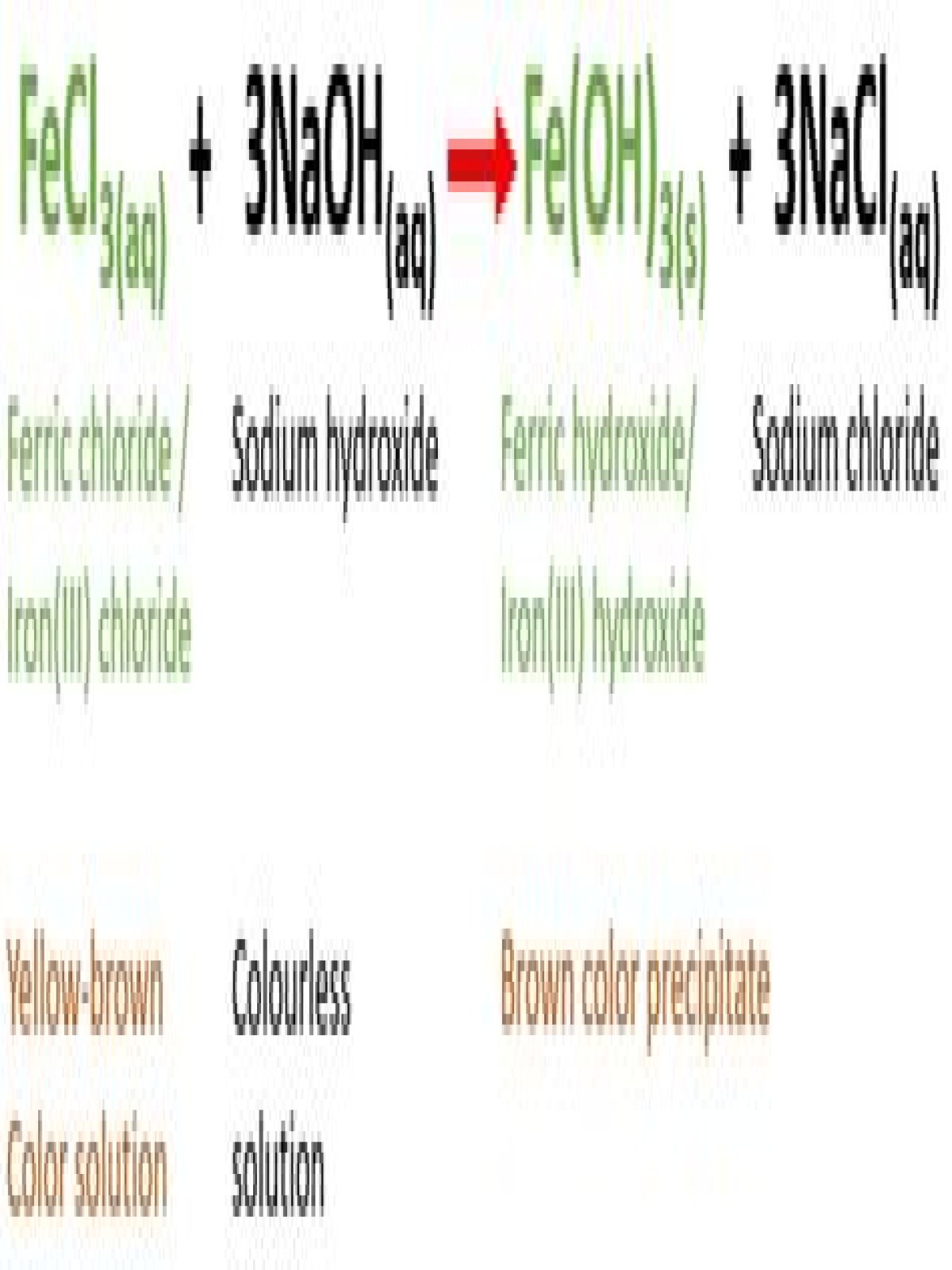
Type of Chemical Reaction: For this reaction we have a double replacement reaction.
Aqueous ferric chloride (FeCl3) reacts with aqueous sodium hydroxide (NaOH) to produce ferric hydroxide ( Fe(OH)3 ) and sodium chloride (NaCl). So, yellow-brown color solution will turn into a brown color precipitate.
Is NaOH FeCl3 an acid base reaction?
The equation for the reaction is: 2NaOH + FeCl2 →Fe(OH)2 (green p.p.t) + 2NaCl. This is a precipitation reaction or double decomposition reaction. That’s a case of some of the very interesting reactions of NaOH, which is as a strong base.
What coefficient goes in front of NaOH?
Balancing the Equation
To balance them, place a coefficient of 4 in front of NaOH.
What type of reaction is ferric hydroxide?
Under anaerobic conditions, the iron(II) hydroxide can be oxidised by the protons of water to form magnetite (iron(II,III) oxide) and molecular hydrogen. This process is described by the Schikorr reaction: 3 Fe(OH)2 → Fe3O4 + H2 + 2 H2O.
What type of reaction is sodium hydroxide and copper sulfate?
The reaction between CuSO4 and NaOH is a double displacement reaction that forms Na2SO4 and Cu(OH)2. Copper (II) hydroxide will precipitate from the solution and appears as a pale blue solid.
Is NaOH an acid or base?
Now let’s look at lye, a strong base with the chemical formula NaOH (sodium hydroxide).
Is nh4f an acid or base?
The aqueous solution is acidic.
Is KClO4 acidic basic or neutral?
(b) Potassium perchlorate, KClO4, is a neutral salt. Neither K+ nor ClO4- has any tendency to donate or accept a proton in dilute aqueous solutions. The reaction between a strong base KOH and the strong acid HClO4 produces KClO4. Its cation, CH3NH3+, is a weak acid.
What is in caustic soda?
Caustic soda is the chemical compound sodium hydroxide (NaOH). This compound is an alkali – a type of base that can neutralize acids and is soluble in water. Today caustic soda can be manufactured in the form of pellets, flakes, powders, solutions and more.
What happens when FeCl3 is treated with excess of NaOH?
If FeCl3 is added to excess of hot water, a positively charged sol pf hydrated ferric oxide is formed due to adsorption of Fe3+ ions. However, when ferric chloride is added to NaOH a negatively charged sol is obtained with adsorption of OH− ions.
What is the observation when ferric chloride is treated with Hydrolysable?
Test with ferric chloride: Add 5 % ferric chloride solution drop by drop to 2-3 ml of the extract and observe the color produced. Hydrolysable tannins (gallitannins and ellagitannins) give bluish-black color or precipitate and condensed tannins brownish-green ones.
What is fe2o3 2H2O?
The compound formed when iron reacts in the air with oxygen is ferric oxide. It has a reddish-brown hue and is also known as the hematite and rust mineral. Ferrous oxide is black and is referred to as the mineral wustite.
Which sol is formed during hydrolysis of FeCl3?
Fe(OH)3 is formed due to hydrolysis of FeCl3 .